
Electroporation is a powerful, highly efficient technique for introducing nucleic acids, proteins, and other molecules into a wide variety of cells. A high-intensity electric field transiently permeabilizes the membrane, enabling uptake of exogenous molecules from the surrounding medium. This technique has been used to introduce nucleotides, RNA, proteins, carbohydrates, dyes, and virus particles into prokaryotic and eukaryotic cells. Electroporation provides a valuable and effective alternative to other physical and chemical methods of transfection.
Since the introduction of our first electroporator in 1986, Bio-Rad has set new standards for electroporation. Now, with the introduction of the Gene Pulser Xcell system, we have raised the standard even higher.
The Gene Pulser Xcell is a modular electroporation system, which includes a main unit, two accessory modules (the CE module and the PC module), and the ShockPod cuvette chamber. The CE module is recommended for use with the Gene Pulser Xcell main unit for electroporation of most eukaryotic cells, including mammalian cells and plant protoplasts. The PC module is recommended for electroporation of bacteria and fungi, as well as other applications where high-voltage pulses are applied to samples of small volume and high resistance. Key features include:
- Provides both exponential and square waveforms
- Electroporates all cell types, prokaryotic and eukaryotic
- Modular design enables a choice of systems
- User-friendly digital interface has easy, intuitive programming — controls all parameters, including those from accessory modules
- Provides a choice of programs for manual operation, preset protocols, user protocols, an optimization protocol, as well as additional advanced functions
- Uses Bio-Rad's patented PulseTrac circuitry and arc protection to ensure reproducibility and sample protection
A Choice of Exponential or Square Waves
Both exponentially decaying and square-wave pulses have been used very effectively for both electroporation and electrofusion. The shape of the electroporation wave can have a significant effect on the transformation efficiency for different cell types. The Gene Pulser Xcell system generates both exponential and square waveforms, enabling you to choose the waveform and protocol that will work best for your cells.

A, exponential decay pulse from a capacitance discharge system. When a capacitor, charged to a voltage Vo, is discharged into cells, the voltage applied to the cells decreases over time in an exponential curve such that the voltage V at any given time t is given by V = Vo e–(t/ RC). In the special case where t = CR, then V = Vo/e. The value CR is known as the time constant of the voltage decay. The shorter the time constant the faster the decay. B, square-wave pulse from a capacitance discharge system. The pulse length is the time the cells are subjected to the discharge. During the pulse, the voltage again decreases by an exponential decay so that at the end of the pulse the voltage is lower than at the beginning. This drop in voltage is called the pulse droop and it is measured as a percentage of the initial voltage.
Exponential decay — The set voltage is released from the selected capacitor and decays rapidly (exponentially) over time (in ms). The delivered pulse is characterized by two parameters, the field strength (in kV/cm) and the time constant (in ms). Adjusting the voltage on the Gene Pulser Xcell for a known electrode distance controls the field strength. Resistance and capacitance can also be set using the Gene Pulser Xcell interface.
Alternatively, by setting the time constant and voltage required, the instrument will set the field strength, resistance, and capacitance values for you. Following the pulse, the instrument will display values for the actual volts delivered and the time constant.
Square wave — For some cell lines, particularly sensitive lines that are easily killed using exponential decay waves, square waves offer increased efficiency and viability. Square-wave pulses are characterized by the voltage delivered, the length of the pulse, the number of pulses, and the length of the interval in between pulses. All of these parameters can easily be set using the Gene Pulser Xcell interface. Following the pulse, the instrument will display the actual volts delivered, the pulse time, and the interval time (when multiple pulses are used).
Reliable, Reproducible, and Safe Performance With PulseTrac Circuitry and Arc Protectio
Bio-Rad's unique, patented microprocessor controlled circuitry delivers reproducible results for any protocol, giving you confidence that the voltage-delivered values can be tested and repeated worldwide, regardless of the media used. PulseTrac and arc protection:
- Facilitate user-selected capacitor recalibration to maintain accurate pulse specification, correcting for capacitor drift that occurs over time
- Provide prepulse sample resistance measurement
- Tighten the already rigorous precision of the low-voltage capacitors in the CE module from 20 to 10%
- Enable safe, automatic discharge of current if the pulse or circuit is interrupted
- Reduce the risk of arcing in the high-voltage circuit, protecting both instrument and sample
User-Friendly Interface Enables Easy Programming and Control of All Functions
The graphical interface on the main unit controls all functions, including those of any connected accessory modules. The interface consists of a single screen used with function keys and an alphanumeric keypad. Programming is simple and intuitive using onscreen prompts. The screen is used for programming, for display of stored programs, set programs, and parameters delivered, and for a graphical display of the waveform. Protocols include:
- Manual programming for entry or editing of all parameters for square-wave or exponential decay
- Assisted programming from the time constant required
- Preset optimized programs for common bacterial, fungal, and mammalian cell lines
- An optimization protocol
- Delivery parameters, including time constant, actual volts applied, pulse interval, and pulse time, depending on the waveform chosen
- Storage and recall of pulse parameters for previous 100 experiments
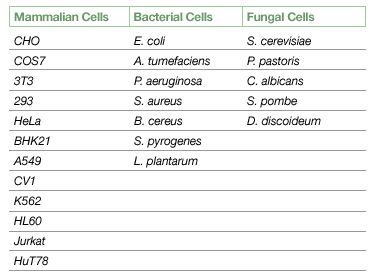
Preoptimized Programs for Rapid Program Selection of Commonly Used Microbial and Mammalian Cells
These programs propose a specific and preoptimized set of parameters for each cell line.
 |
Gene Pulser Xcell eukaryotic system (catalog #165–2661) — Consisting of the main unit and the CE module, the eukaryotic system enables electroporation of mammalian cells and plant protoplasts. With a range of 25–3,275 µF, the CE module provides a means of controlling the capacitance of the circuit by increasing the time constant of the pulse. For square-wave pulses, the CE module provides the large capacitor necessary for delivering a square-wave pulse into low-resistance media. |
ShockPod Shocking Cuvette Chamber — Designed for One-Handed Use to Allow Easier, More Rapid Sample Handling
The innovative ShockPod chamber enables rapid and easy one-handed operation with a single cuvette. A simple catch on the front of the ShockPod opens the lid. The cuvette slot is designed so that the cuvette may only be inserted in the proper orientation.

The ShockPod has a safety interlock that prevents a pulse being delivered to the cuvette when the ShockPod lid is open.
Specifications
|
Output Waveform Voltage |
Exponential decay or square wave 10–3,000 V |
| Capacitance |
10–500 V, 25–3,275 µF in 25 µF increments 500–3000 V, 10, 25, 50 µF |
| Sample resistance |
20 ohm minimum at 10–2,500 V 600 ohm minimum at 2,500–3,000 V |
| Square-wave timing |
10–500 V: 0.05–10 ms duration with 0.05
ms increments, 10–100 ms duration with 1 ms increments, 1–10 pulses,
0.1–10 sec interval 500–3,000 V: 0.05–5 ms duration with 0.05 ms increments, 1–2 pulses, 5 sec minimum interval |
| Input voltage | 100–120 VAC or 220–240 VAC, 50/60 Hz |
| Power | Max. 240 W (during short charging periods) |
| Operating environment | Temp. 0–35°C, humidity 0–95% (noncondensing) |
| Regulatory |
Safety EN 61010, EMC EN61326 Class A |
|
Dimensions and weight Main unit CE module PC module |
31 x 30 x 14 cm, 6.6 kg 31 x 28 x 9 cm, 3.1 kg 31 x 28 x 5 cm, 1.9 kg |